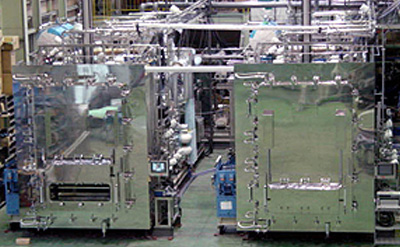
SOLUTIONSFreeze-Drying Solution
KYOWAC is continuously conducting technical studies on freezing, vacunm, sterilization, and drying.
- Comprehensive support of technical hardware/software 【Total produce system 】
- KYOWAC is a specialized producer of freeze dryers. We are the only company in the world that handles all freeze dryers, including those for testing, pharmaceutical, and foods applications. In the half century since our foundation, in addition to production, we have been engaged in technical reporting and activities which include the Japanese Society For Cryobiology and Cryotechnology, the Japanese Society of Pharmaceutical Machinery and Engineering, Parenteral Drug Association–Japan Chapter, International Society for Pharmaceutical Engineering, and others. Serving as a provider of comprehensive solution services, our expertise encompasses both mechanical hardware and product drying program software.
Detailed response for customer software needsDrying program development/analytical support

Our testing labs, equipped with facilities that are always ready to conduct freeze-drying testing, are staffed with specialists who provide detailed support.
- 01 Collapse temperature measurement
- 02 Test drying with compact test dryer for pharmaceuticals and foods.(0.4㎡).
- 03 Scale-up testing using a compact production machine (2㎡) for pharmaceuticals
- 04 Test drying with ICS tube-type equipment
We provide drying programs based on drying results. We also offer scale-up programs for the customer’s production equipment or contract manufacturer. Our full-time staff is ready to respond at any time to questions related to freeze-drying or drying. Moreover, we have begun joint research and development into consultation on product appearance improvement, time reduction, etc.
01.Collapse temperature settingFirst-time customers
About collapse temperature
The collapse temperature is a crucial temperature for freeze-drying. Every solute has a characteristic temperature. If the temperature of a frozen portion rises above this temperature during freeze-drying, this portions will begin to contract and collapse, resulting in dissolution and foaming, and failure of freeze-drying.
A freeze-drying program can be created based on information that includes the collapse temperature, solid content concentration, and thickness of the frozen part, etc.
- Measurement of the collapse temperature (Tc) under the Clio microscope(example of 10% sucrose)
-

Normal dry state 
Collapse generating state
02.Dry process analysisCustomers already possessing a dryer
Please send the collapse temperature, quantity currently divided and poured out, solid content concentration, vial container dimensions, and current drying chart paper to our test-and-research lab.
We will analyze the drying chart and propose an optimal drying program.
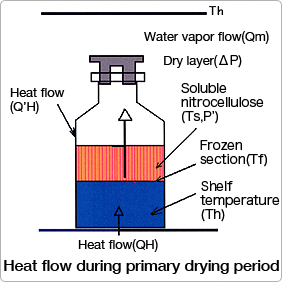
03.Optimal setup of a production machineFor the customer who is considering production machine purchase
Please send the collapse temperature, quantity currently divided and poured out, solid content concentration, vial container dimensions, and the drying program of a your testing machine to our test-and-research lab. The drying chart is analyzed, and, incorporating a scale-up factor, we will propose an optimal drying program.
【Chart on the right 】 A geometric difference exists between a testing machine and production machine. We set up an optimal program based on an analysis of heat transfer from shelf to central vial and analysis of radiant heat transfer from wall to shelf end and to central vial.
In-Plant SeminarsSeminars

SeminarsService Info. Seminars covering various topics related to freeze-drying will be conducted by industry organizations. Implant seminars held by customers will also be actively conducted. Please do not hesitate to request us.
From the fundamentals of freeze-drying to state-of-the-art technology
KYOWAC engages in technological issues related to the needs of the times through constant detailed study of the fundamental technologies of freezing, vacuum, and sterilization. Technical resources developed in this way are not only applied to products, but also widely deployed in the forms of technical reporting, introduction support, and educational activities.
- Seminar contents
- Please feel free to inquire about other topics.
- 01 Fundamentals of freeze-drying
- 02 Freeze-drying process and validation
- 03 Analysis of freeze-drying process and creation of optimal program
- 04 Scale-up technology for testing equipment to production equipment
- 05 In-place cleaning of freeze-drying machine
- 06 Chamber enter/exit system for freeze-drying machine for sterile injectables
- 07 Accident/failure analysis of the freeze-drying process through a risk base approach
- 08 Trends in state-of-the-art technology of freeze-drying in the manufacture of pharmaceuticals
- 09 ICS/FD (Integrated Closed System)

Particualy important after purchase serviceMaintenance service

Freeze dryers are the result of putting together canning items, such as drying chambers, shelves, cold traps, and the like, and such items as refrigerators, shelves, vacuum pumps, brine pumps. KYOWAC is a specialist in freeze dryer diagnostic equipment. Failure factors are analyzed, and in cooperation with the maintenance departments of individual equipment specialist manufacturers, repair and parts replacement are carried out. The maintenance history of equipment manufactured by us is recorded in a maintenance file, which is kept until the equipment is disposed of.
An expert group that diagnoses equipment
KYOWAC is a specialist in freeze dryer diagnostic equipment. Failure factors are analyzed, and in cooperation with the maintenance departments of individual equipment specialist manufacturers, repair and parts replacement are carried out. To maintain equipment in its best condition, we ask our customers to conduct periodic inspections and sign a maintenance contract. KYOWAC is a specialist in freeze dryer diagnostic equipment. The maintenance staff, comprising highly knowledgeable personnel with extensive experience in the manufacture of freeze-drying machines, covers the entire country from their bases in Saitama and Osaka. Failure factors are analyzed, and in cooperation with the maintenance departments of individual equipment specialist manufacturers, repair and parts replacement are carried out.

A detailed after-purchase system
To maintain equipment in its best condition, we ask our customers to conduct periodic inspections and sign a maintenance contract. Equipment maintenance and meter calibration are carried out. The maintenance history of equipment manufactured by us is recorded in a maintenance file, which is kept until the equipment is disposed of. KYOWAC acquired ISO 9001-2000 and is working with the aim of improving customer satisfaction receipt of order to design, manufacture, delivery, and maintenance. We also perform modification such as equipment upgrading and moving of equipment.

Supports the GMPs and validations of Japan, the U.S., and Europe.Validation support
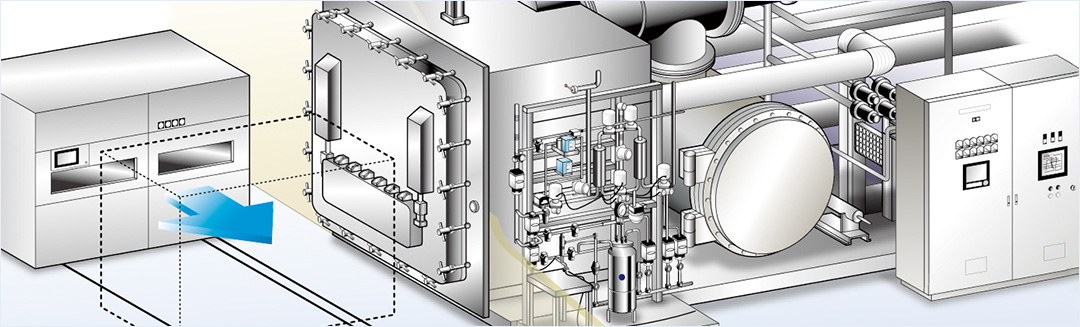
The freeze-drying process requires sophisticated technology; guaranteeing it requires a wealth of knowledge and experience. We are engaged in the creation of validation documents for freeze-drying manuals for development to production, for contract manufacturers, and for drying program development.
About validation
Validation refers to verifying through documentation that a product manufactured by the concerned process always complies to set standards and that the product is firmly guarantees having specified quality attributes.
Regarding development manufacturing process parameters (CPP) that determine critical quality attributes (CQA), we develop combinations that can be widely applied within the design space and provide documentation support for application forms. We also are engaged in the creation of validation documents for freeze-drying manuals from development to production and for contract manufacturers, and for program development.
- 3 steps in drug development
-
(Storage stability)
- ① Determine the standard of Critical Product Quality (CPQ) about the product
(Moisture content)
- ② Determine Critical Quality Attributes (CQA) to meet the CPQ
Manufacturing process(Shelf temp, Pressure, Drying time)
- ③ Define the Critical Manufacturing Process / Critical Process Parameter (CPP) to determine the CQA
Reliable supporting technologies for sterile injection manufacture
We manufacture the equipment whose technical specifications comply with sterile injectable manufacture standards that support the GMP of Japan, the USA, and Europe. Many optional specifications, such as main door/ small door, CIP, SIP, and an automatic loading system, are available.